The mechanism by which oxygen-deficient combustion inhibits the conversion of organic nitrogen to NOx is crucial for optimizing Low NOx Burner performance and designing efficientIndustrial Combustion Systems. This article details the core principles and their practical applications in key industrial scenarios such as waste incineration, sludge drying, and exhaust gas treatment—aligning with the operational needs of Industrial Burners and customized combustion solutions.
1. Conversion Pathways of Organic Nitrogen in Industrial Combustion
Organic nitrogen (present in fuels for Industrial Drying Combustion System, Industrial Waste Incineration Combustion System, etc., such as proteins and nitrogen-containing heterocyclic compounds) first pyrolyzes or oxidizes into intermediate products at high temperatures. These intermediates, mainly HCN (hydrogen cyanide) and NH₃ (ammonia), are called “nitrogen precursors” and are central to NOx formation in High-temperature Burner and Direct-Fire Burner operations. Subsequently, the precursors follow two competitive conversion pathways:
-
Oxidation pathway: Under oxygen-rich conditions (common in traditional Air Heating Burner and non-optimized combustion systems), HCN/NH₃ reacts with free radicals such as O₂, O•, and OH•, eventually generating NOx (mainly NO)—a major emission concern for industrial heating applications.
-
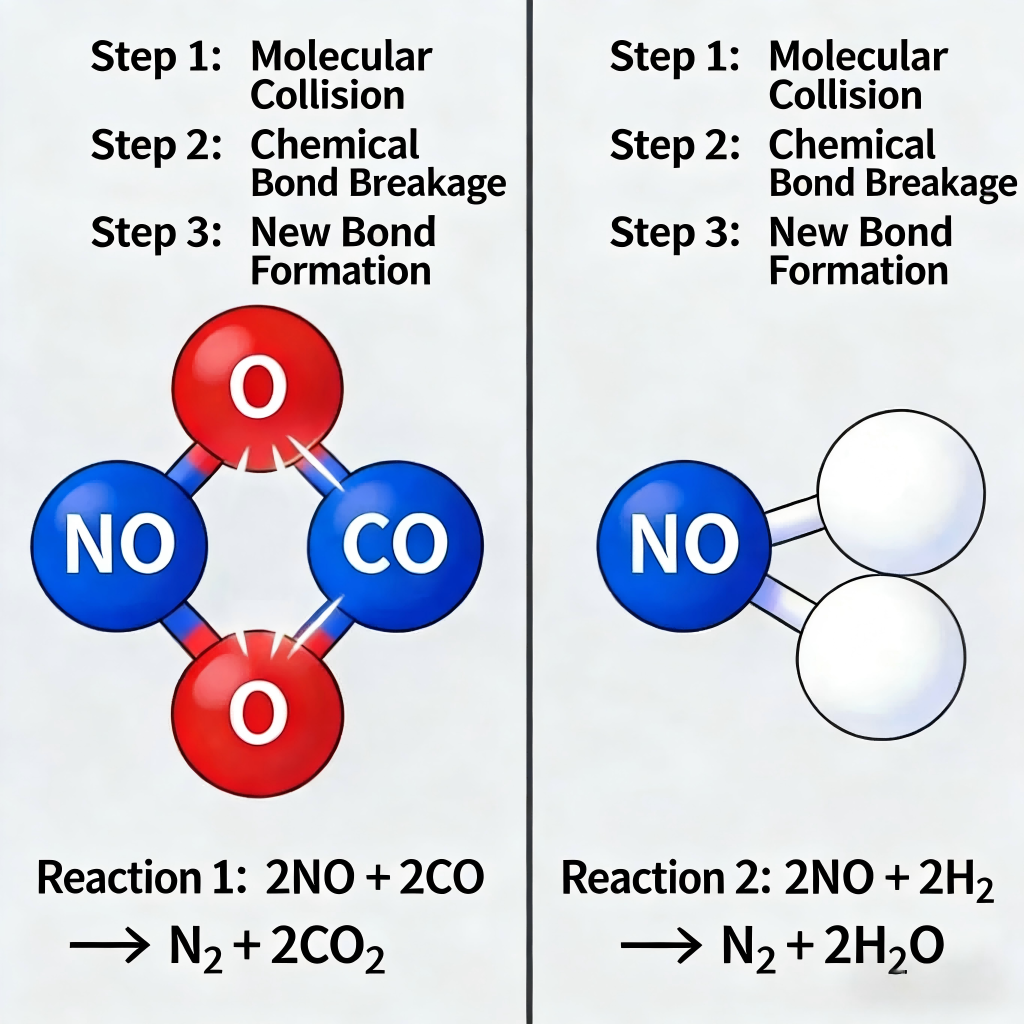
Reduction pathway: Under oxygen-deficient or reducing atmospheres (a key design feature of Low NOx Burner), HCN/NH₃ reacts with NO, CO, H₂, etc., or directly converts nitrogen atoms into N₂—achieving low-emission combustion for systems like Industrial Sludge Drying Combustion System and Exhaust Gas Incineration Burner.
2. Key Role of Oxygen-Deficient Environment in Low NOx Industrial Burners
Oxygen-deficient (hypoxic) conditions—engineered into Low NOx Burner and Industrial Regenerative Combustion System designs—inhibit NOx generation through three core mechanisms:
(1) Inhibiting Oxidation of Nitrogen Precursors for Low NOx Emissions
In oxygen-deficient combustion environments (optimized for High-Speed Burner and Self-Recuperative and Radiant Tube Burners), the concentration of oxidative free radicals (such as O• and OH•) significantly decreases. This weakens the reaction between HCN/NH₃ and oxygen, directly reducing NO formation—a critical advantage for meeting emission standards in Industrial Hazardous & Solid Waste Treatment Combustion System.
Key Reaction Example for Industrial Applications:
Oxygen-rich (traditional burners):
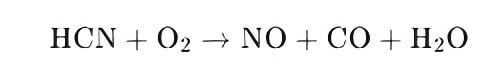
(promotes NOx formation, a challenge for non-low-NOx Line&Duct Burners and Immersion Tube Burner without oxygen control).
Oxygen-deficient (Low NOx Burner design): This reaction is suppressed, and HCN shifts toward the reduction pathway—aligning with the emission goals of Industrial Exhaust Gas Incineration Burner System and Industrial Calcination Kiln Combustion System.
(2) Promoting Reductive Reactions in Customized Combustion Systems
Oxygen-deficient conditions in Customized Combustion System (e.g., Industrial Marine Heating Combustion System and Industrial Rotary Kiln Combustion System) generate reductive substances such as CO, H₂, and hydrocarbon free radicals. These substances reduce already formed NO to N₂ or directly convert precursors into N₂—enhancing the low-NOx performance of High-temperature Burner and Direct-Fire Burner:
-
NO reduction reaction:
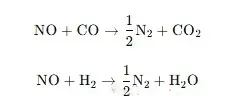
-
Direct reduction of precursors:
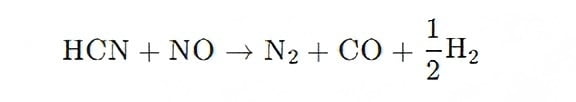
(3) Lowering Combustion Temperature for Stable Burner Operation
Oxygen-deficient combustion, a key feature of Self-Recuperative and Radiant Tube Burners and Industrial Regenerative Combustion System, is usually accompanied by a local temperature decrease. This inhibits the generation of “thermal NOx” (Zeldovich mechanism)—beneficial for protecting components of High-pressure High-temperature Valve & Piping System (Skid-mounted) in high-temperature applications. Note that organic nitrogen conversion mainly produces “fuel NOx,” so temperature influence is relatively secondary for Industrial Drying Combustion System and Industrial Ignition Combustion System.
3. Competitive Pathways of Intermediate Products in Low NOx Burners
In oxygen-deficient environments (designed for Low NOx Burner and Customized Combustion System), the conversion of HCN/NH₃ favors harmless pathways that align with emission requirements for Industrial Waste Incineration Combustion System and Industrial Sludge Drying Combustion System:
HCN → NCO (cyanate radical) → NH₃ → N₂
Or directly capture nitrogen atoms through reductive substances such as CH• and C to form N₂—an optimized process in High-Speed Burner and Air Heating Burner designs.
4. Practical Control Strategies for Industrial Burners & Combustion Systems
In industrial applications (e.g., Hot Air Furnace, Direct-fired Hot Air Heater, Indirect-fired Hot Air Heater), “staged combustion” or “low-oxygen combustion” technologies are widely adopted in Low NOx Burner and Customized Combustion System to leverage the above mechanism:
-
First stage (oxygen-deficient zone): Control air supply (air-fuel ratio lower than the stoichiometric ratio) in Industrial Burners (e.g., Line&Duct Burners, Immersion Tube Burner) to convert organic nitrogen into N₂ in a reducing atmosphere—critical for Industrial Hazardous & Solid Waste Treatment Combustion System.
-
Second stage (burn-out zone): Supplement remaining air to completely oxidize unburned substances. This stage is integrated with Industrial Electronic Ratio Combustion Control System to ensure efficiency, and since nitrogen is already fixed as N₂, NOx generation is greatly reduced—ideal for Industrial Exhaust Gas Incineration Burner System and Industrial Calcination Kiln Combustion System.
5. Core Mechanism of NOx Inhibition by Oxygen Deficiency for Industrial Combustion
|
Conditions
|
Main Pathway
|
Final Product
|
Relevance to Industrial Burners
|
|---|---|---|---|
|
Oxygen-rich combustion
|
Oxidation-dominated (HCN/NH₃ → NO)
|
Increased NOx
|
Traditional Industrial Burners (high emission risk)
|
|
Oxygen-deficient combustion
|
Reduction-dominated (HCN/NH₃ → N₂)
|
Mainly N₂, reduced NOx
|
Low NOx Burner, Industrial Regenerative Combustion System (optimized design)
|
Key point: Oxygen-deficient environments—engineered into Customized Combustion System and Low NOx Burner—”lock” nitrogen in N₂ by (1) reducing oxidative free radicals; (2) providing a reducing atmosphere; (3) promoting NO reduction reactions. This is the core principle for meeting emission standards inIndustrial Waste Incineration Combustion System, Industrial Sludge Drying Combustion System, and other high-demand applications.
6. Practical Applications & Product Alignment
-
Target NOx Type: This mechanism mainly addresses “fuel NOx” (derived from fuel nitrogen), the primary emission source for Industrial Drying Combustion System, Industrial Ignition Combustion System, and Exhaust Gas Incineration Burner.
-
Operational Precision: Practical operation requires precise control viaIndustrial Electronic Ratio Combustion Control System to adjust the degree of oxygen deficiency, avoiding secondary pollution (e.g., CO, dioxins) in Industrial Hazardous & Solid Waste Treatment Combustion System. This also relies on reliable Customized Gas Piping / Valve Train System (Skid-mounted) and Customized Explosion-proof Piping / Valve System (Skid-mounted) for stable air-fuel control.
-
Key Product Applications: In Industrial Waste Incineration Combustion System, Industrial Rotary Kiln Combustion System, andHot Air Furnace, our Low NOx Burner, Self-Recuperative and Radiant Tube Burners, and Customized Combustion System leverage this mechanism to achieve low emissions. For special scenarios, Customized LNG / Methanol Fuel Supply Piping & Valve System (LFSS Skid-mounted) and High-pressure High-temperature Valve & Piping System (Skid-mounted) ensure compatible, safe operation with oxygen-deficient combustion.